"16. Being a reliable source of market research information, North America In-Vitro Toxicology Testing Market reports expand the reach of business success. This market analysis provides knowledge about various segments that are relied upon to observe the fastest business development within the framework of forecast estimates. By thinking from the end user's point of view, a team of researchers, forecasters, analysts and industry experts worked meticulously to formulate this market research report. The use of proven tools such as SWOT analysis and Porter's Five Forces analysis is very useful in creating a superior North America In-Vitro Toxicology Testing Market report.
The worldwide North America In-Vitro Toxicology Testing Market business report examines the market by region, especially North America, China, Europe, Southeast Asia, Japan, and India, focusing on the leading manufacturers in the global market, with respect to production, price, revenue and market share for every manufacturer. This is useful for knowing the general conditions prevailing in the market, the market and competitors' pricing strategies. Some of the prominent features used while creating this market research report include the highest level of passion, practical solutions, committed research and analysis, modernism, integrated approach, and latest technology. To get detailed North America In-Vitro Toxicology Testing Market reports, request an analyst call or submit an inquiry any time.
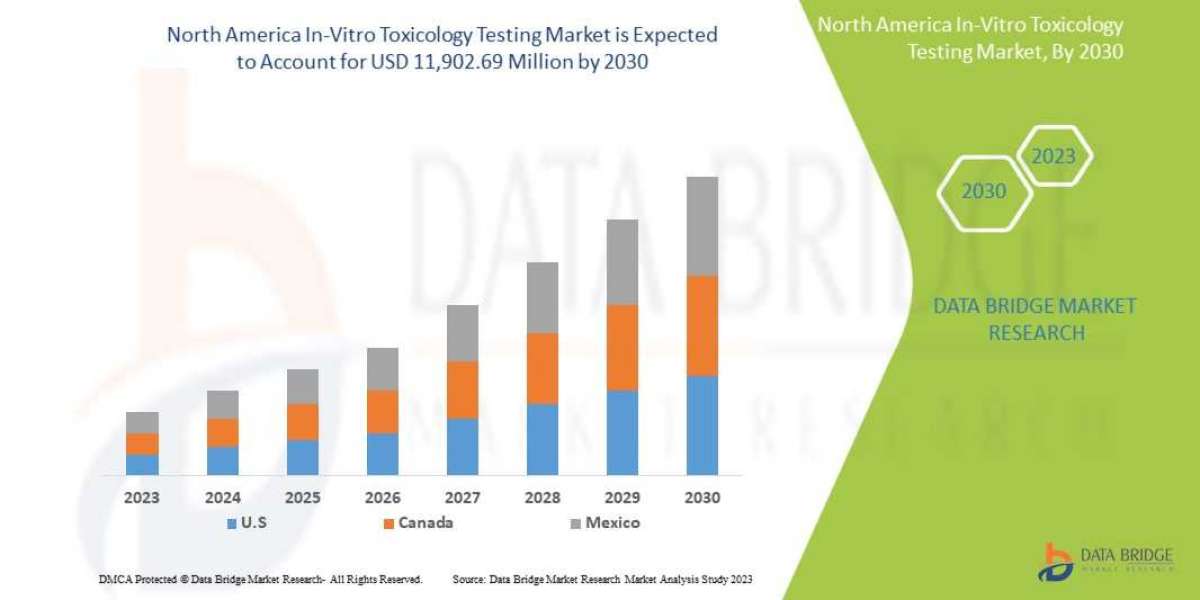
Data Bridge Market Research analyzes that the North America in-vitro toxicology testing market is expected to reach USD 11,902.69 million by 2030, at a CAGR of 14.0% during the forecast period.
Explore Further Details about This Research North America In-Vitro Toxicology Testing Market Report https://www.databridgemarketresearch.com/reports/north-america-vitro-toxicology-testing-market
REPORT METRIC | DETAILS |
Forecast Period | 2023 to 2030 |
Base Year | 2022 |
Historic Years | 2021 (Customizable to 2015 - 2020) |
Quantitative Units | Revenue in USD Million |
Segments Covered | Product and Service (Consumables, Services, Assays, Equipment’s, and Software), Toxicology End Point and Test (ADME (Absorption, Distribution, Metabolism, Excretion) Testing, Cytotoxicity Testing, Genotoxicity Testing, Dermal Toxicity Testing, Ocular Toxicity Testing, Organ Toxicity Testing, Skin Irritation, Corrosion, Sensitization Testing, Phototoxicity Testing, and Other Toxicity Endpoints Tests), Technology (Cell Culture Technologies, High-Throughput Technologies, Molecular Imaging, and OMICS Technology), Method (Cellular Assays, Biochemical Assays, Ex-Vivo Models, and In Silico Models), Industry (Pharmaceutical Biopharmaceutical Companies, Diagnostics, Food, Chemicals, Cosmetics Household Products), Distribution Channel (Direct Tender, Retail Sales, and Others) |
Countries Covered | U.S., Canada and Mexico |
Market Players Covered | Thermo Fisher Scientific Inc., Labcorp Drug Development, Merck KGaA, Charles River Laboratories, Lonza, Bio-Rad Laboratories, Inc., Catalent, Inc, SGS Société Générale de Surveillance SA, QIAGEN, Intertek Group plc., Eurofins Scientific, Promega Corporation, Aragen Life Sciences Ltd., Cyprotex Plc., Shanghai Medicilon Inc., Creative Biolabs, BioIVT, AAT Bioquest, Inc., Gentronix, IONTOX, InSphero, MB Research Laboratories, Creative Bioarray, and Preferred Cell Systems |
Market Definition
In-vitro toxicology testing involves testing chemicals, drugs, and other substances to assess their potential toxicity using in-vitro (non-animal) testing methods. In-vitro toxicology testing involves using cells, tissues, or cellular components outside their natural environment to evaluate substances' safety and potential hazards. The market encompasses a wide range of in-vitro tests and assays that assess various aspects of toxicity, including but not limited to cytotoxicity, genotoxicity, carcinogenicity, organ toxicity, reproductive toxicity, and environmental toxicity. These tests are conducted on cell cultures, tissue models, or other in-vitro systems to mimic the response of biological systems to potential toxicants.
North America In-Vitro Toxicology Testing Market Dynamics
This section deals with understanding the market drivers, advantages, opportunities, restraints, and challenges. All of this is discussed in detail below:
Drivers
Increasing Demand for Toxicology Testing Products
The main activity of the pharmaceutical industry is quality management. Drugs must be sold as formulations that are sterile, therapeutically active, reliable, and predictable in their performance. New and improved medicinal agents are being developed at an accelerated speed. At the same time, more exact and sophisticated analytical methods are being developed for their evaluation. Due to the rising incidence of chronic diseases and the emergence of the COVID-19 pandemic, the demand for drugs and medical devices has proportionally increased. The demand for toxicology testing further gets escalated with the increased demand for the products of biotechnology and pharmaceutical industries,
A toxicology study is a crucial part of drug development used to characterize the toxicity profile of a drug by identifying its impact on organ structure/ functionality. The study provides critical information and knowledge that is used by regulatory agencies, among others to prevent or reduce the likelihood that a disease or other negative health outcome would occur. The Food and Drug Administration has issued several guidance documents for industry such as safety testing of drug metabolites, in-vitro metabolism and transporter-mediated drug-drug interaction studies, and clinical drug interactions studies.
Rapidly Growing Pharmaceutical and Medical Device Industries
Many revolutionary trends and advances have been witnessed by the pharmaceutical and medical device industries that have dramatically improved the medicines available to patients. It was possible to witness the effect of artificial intelligence and big data on the diagnosis and treatment of diseases.
Combined with their potential to remedy previously untreatable diseases, the effectiveness and protection of biopharmaceutical drugs help pharmaceutical companies to succeed. An opportunity for sustained healthy growth supports the existing biologics-development pipeline. Since 1995, the number of biotech patents applied has increased by 25% annually. More than 1,500 biomolecules are presently undergoing clinical trials, and the biologics success rate has so far been more than double that of small-molecule drugs, with 13% of biopharmaceuticals entering the phase I testing phase going on to launch.
The prosperity and growth of these industries proportionally increase the demand for toxicity testing for the quality control of the products developed by them. Therefore, the rapidly growing pharmaceutical and medical device industries act as a driver for the growth of the market.
Opportunity
Growing Adoption of Quality Check Products to Prevent Product Recalls
Medical devices, drugs, and biologics are playing an increasingly important role in today's healthcare delivery. However, ongoing quality concerns with drugs and related recalls raise possible health hazards to the use of these drugs on patients. One of the major reasons for product recall in the pharmaceutical companies is the toxicity profile of their drugs and other products which could pose serious health complications among the patients who consume them. There has been detailed reporting of the potentially devastating effects of toxicity on humans. It is common for toxins to trigger permanent side effects at sufficient concentrations throughout the bloodstream and even death in extreme cases.
In-process controls to track the existence of toxicity must include the aseptic production of compounded products. Therefore, in the pharmaceutical and biomedical industries, effective and efficient monitoring for the existence of toxicity is important. All injectable or implantable products labelled as pyrogen-free or sterile must undergo toxicology testing before being released. This avoids toxicity in patients and makes it easy to comply with regulatory and cGMP guidelines. Hence, the growing adoption of quality check products to prevent product recalls acts as an opportunity for the growth of the market.
Key Offerings:
- Past Market Size and Competitive Landscape (2018 to 2022)
- Past Pricing and price curve by region (2018 to 2022)
- Market Size, Share, Size Forecast by different segment | 2023−2029
- Market Dynamics – Growth Drivers, Restraints, Opportunities, and Key Trends by Region
- Market Segmentation – A detailed analysis by segment with their sub-segments and Region
- Competitive Landscape – Profiles of selected key players by region from a strategic perspective
- Competitive landscape – North America In-Vitro Toxicology Testing Market Leaders, Market Followers, Regional player
- Competitive benchmarking of key players by region
- PESTLE Analysis
- PORTER’s analysis
- Value chain and supply chain analysis
- Legal Aspects of Business by Region
- Lucrative business opportunities with SWOT analysis
- Recommendations
Browse Related Reports:
Peripartum cardiomyopathy Market Size, Share, Growth | Opportunities,
Europe Bone Densitometer Devices Market Size, Share Analysis Report
Polysaccharides and Oligosaccharides Market Size, Share, Growth Analysis
Molecular Cytogenetic Systems Market Size, Share, Growth
Point-to-Point (P2P) Antennas Market Size, Share Trends: Report
Human granulocytic ehrlichiosis Market Size, Share, Industry, Forecast
Racing Games Market Size, Share Trends [Report]
About Data Bridge Market Research:
US: +1 888 387 2818
UK: +44 208 089 1725
Hong Kong: +852 8192 7475
Email – corporatesales@databridgemarketresearch.com
"