All research and analysis data in the credible Europe e-Clinical Solutions Market report is mapped into an actionable model, with strategic recommendations from experts. Client needs are well understood by experts who utilize their expertise and strong knowledge base to identify and evaluate competition and develop strategic programs, with both short-term and long-term goals. This industry analysis report recognizes and analyzes emerging trends along with key drivers, challenges, and opportunities in the Europe e-Clinical Solutions Market industry. The winning Europe e-Clinical Solutions Market Report provides extensive statistical analysis of the market's ongoing positive development, capacity, production, production value, costs/profits, supply/demand, and import/export
Businesses today prefer market research reports like Europe e-Clinical Solutions Market reports as they help in making better decisions, generating more revenue, prioritizing market goals and achieving profitable business. This market report contains chapters on the global market and related companies along with their profiles, which provide important data regarding their insights in terms of finances, product portfolios, investment plans, and market and business strategies. Exploitation of existing statistical tools and coherent models for market data analysis and forecasting makes the Europe e-Clinical Solutions Market research report perform better.
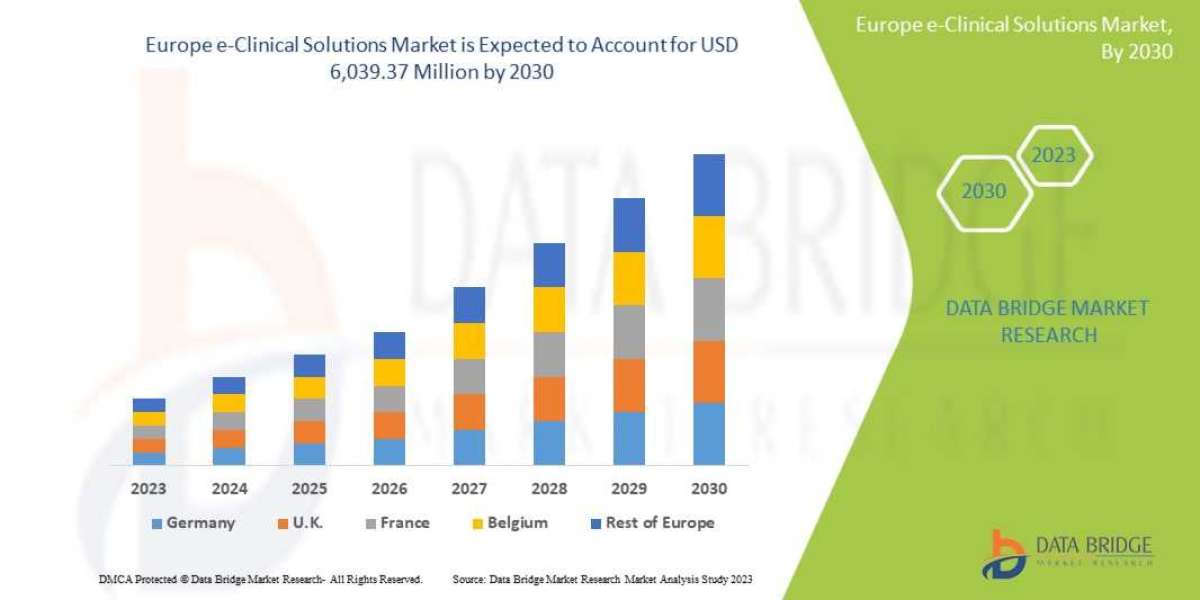
Data Bridge Market Research analyzes that the Europe e-clinical solutions market is expected to reach a value of USD 6,039.37 million by 2030, at a CAGR of 13.3% during the forecast period. This market report also covers pricing analysis and technological advancements in depth
Explore Further Details about This Research Europe e-Clinical Solutions Market Report https://www.databridgemarketresearch.com/reports/europe-e-clinical-solutions-market
.
REPORT METRIC | DETAILS |
Forecast Period | 2023 to 2030 |
Base Year | 2022 |
Historic Years | 2021 (Customizable to 2015 – 2020) |
Quantitative Units | Revenue in USD Million, Volumes in Units, and Pricing in USD |
Segments Covered | By Product (Electronic Data Capture and Clinical Trial Data Management Systems, Clinical Trial Management Systems, Clinical Analytics Platforms, Care Coordination Medical Records (CCMR), Randomization and Trial Supply Management, Clinical Data Integration Platforms, Electronic Clinical Outcome Assessment Solutions, Safety Solutions, Electronic Trial Master File Systems, Regulatory Information Management Solutions, and Others), Delivery Mode (Web-Hosted (On-Demand) Solutions, Licensed Enterprise (On-Premises) Solutions, and Cloud-Based (SAAS) Solutions), Clinical Trial Phase (Phase I, Phase II, Phase III, and Phase IV), Organization Size (Small Medium and Large), User Device (Desktop, Tablet, Handheld PDA Device, Smart Phone, and Others), End User (Pharmaceutical and Biopharmaceutical Companies, Contract Research Organizations, Consulting Service Companies, Medical Device Manufacturers, Hospitals, and Academic Research Institutes) |
Countries Covered | Germany, France, U.K., Italy, Spain, Netherlands, Russia, Switzerland, Turkey, Belgium, and the Rest of Europe, |
Market Players Covered | Oracle, Signant Health, MaxisIT, Paraxel International Corporation, Dassault Systemes, Clario, Mednet, OpenClinica, LLC, 4G Clinical, Veeva Systems, Saama Technologies, LLC, Anju, Castor, Medrio, Inc., ArisGlobal, Merative, Advarra, eClinical Solutions, LLC, Y-Prime LLC, RealTime Software Solutions LLC, Quretec, Research Manager, Datatrack Int., and IQVIA Inc. |
Market Definition
The goal of e-clinical solutions is to revolutionize the field of clinical research. The clinical management organization is trying to improve the effectiveness, efficiency, and accessibility of clinical research data to treat patients more quickly. The idea behind the creation of eClinical Solutions was to pinpoint the problems with clinical research data, fix them, and offer creative solutions to make clinical trial data useful and simple to obtain, as well as to facilitate the standardization, reporting, and operation of the clinical research domain. The rising adoption and growing focus towards the e clinical solutions and software are expected to drive market growth.
The e-clinical solutions, such as electronic data capture (EDC), electronic patient-reported outcomes (ePRO), clinical trial management systems (CTMS), and electronic trial master file (eTMF) systems, are software programs used in clinical research to manage clinical data. However, strict regulations and standards for the approval of clinical trial studies and product approvals are expected to restrain market growth.
Europe e-Clinical Solutions Market Dynamics
This section deals with understanding the market drivers, opportunities, restraints, and challenges. All of this is discussed in detail below:
DRIVERS
Increasing RD activities and corresponding expenditure
Research development is crucial in all industries, including life science and healthcare. The life science industry carries out extensive research and development to generate revenue and often brings results that embrace saving or enhancing a patient's life. Drug development requires going through clinical trials to check clinical data analysis and review its study and new data insights.
The e-clinical solutions are used for comprehensive data review, automating the data transformation process, and analytics to accelerate timelines. Pharmaceutical and biopharmaceutical companies conduct many clinical trials to complete successful drug or biologic development. Pharmaceutical and biopharmaceutical companies have increased their focus on research and development, raising the demand for e-clinical solutions.
Improved healthcare infrastructure and establishment of advanced laboratories
Infrastructure is a key pillar supporting the fundamental aim of promoting improved standards of care and well-being for all patients, together with a good healthcare system experience. In parallel, the healthcare system and staff must support effective health promotion, prevention, and self-care of the whole population.
Infrastructure must integrate the hospital, as the acute and in-patient care center, into the broader healthcare system. It should facilitate the seven domains of quality patient experience, effectiveness, efficiency, timeliness, safety, equity, and sustainability. Infrastructure includes the built environment and supporting elements such as equipment, access, information technology (IT), systems and processes, sustainability initiatives, and staff. This improved healthcare infrastructure and staff more opportunities for e-clinical solutions to grow and give better results.
OPPORTUNITY
Increasing investments by various governments in clinical trials
Clinical trial funding comes from various sources, including the government, commercial investors, nonprofit organizations, academic institutions, and other research organizations. Historically, the National Institutes of Health (NIH) has made the biggest government investments in fundamental drug development research. The defense advanced research agency (DARPA) has also contributed to the discovery stage by accepting a few relatively high-risk biologic initiatives. State governments are also increasingly taking the lead in this area, partly due to the public's frustration with the lengthy discovery process. Thus, government initiatives and investments toward discovering new drugs with trial studies are expected to create an opportunity for market growth.
Key Insights Of Europe e-Clinical Solutions Market Report
- The report comprises a detailed analysis of different segments and offers market valuations between 2023 to 2030.
- This study presents the analytical depiction of the Europe e-Clinical Solutions Market with the current trends and future estimations to determine the imminent investment pockets
- The report also reveals information with respect to key drivers, restraints, and opportunities coupled with a comprehensive analysis of the global Europe e-Clinical Solutions Market
- The forecast period of the Europe e-Clinical Solutions Market is assessed from 2024 to 2030 to highlight the Europe e-Clinical Solutions Market growth scenario.
- Porter’s five forces analysis establishes the effectiveness of the buyers and suppliers in the business line.
- The key players in the industry are profiled to gain an understanding of the strategies adopted by them.
- This report provides the current trends and future estimations during the forecast period, which in turn aids in identify the prevailing market opportunities.
The company portfolio includes company synopsis, operating business sectors, business overview, product/service categories, and recent growth strategies adopted by them.
Browse Related Reports:
Asia-Pacific Espresso Coffee Market Share Statistics Report,Size, Forecast, Trends
Middle East and Africa Digital Payment Market Size, Global Industry Share, Recent
Digital Holographic Microscopy Market by Size, Share, Forecasts, Trends
Merkel cell carcinoma Market Size | Statistics Report, Share, Forecast, Trends
Intelligent Transport System Market Size, Share Trends Analysis Report
Cell Culture Media Market Size and Forecasts, Share and Trends
Lyocell Fiber Market Size, Industry Share, Forecast
About Data Bridge Market Research:
US: +1 888 387 2818
UK: +44 208 089 1725
Hong Kong: +852 8192 7475
Email – corporatesales@databridgemarketresearch.com